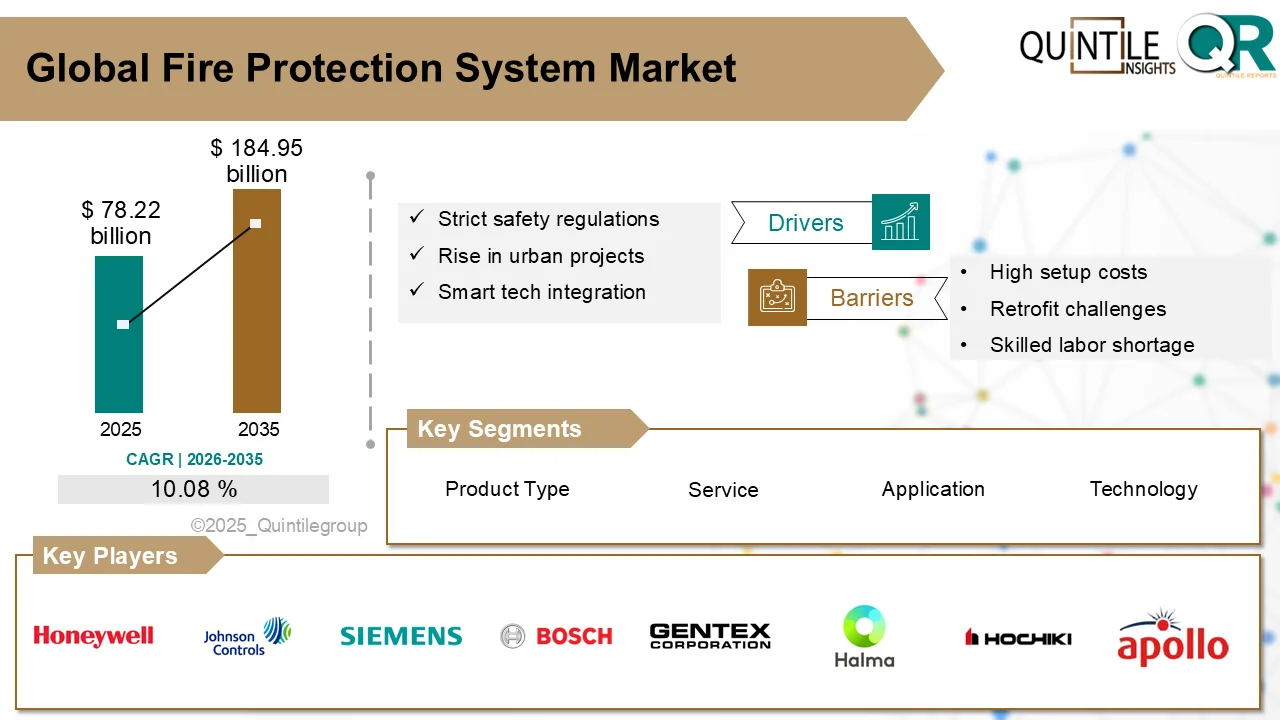
The global pharmacovigilance market is entering a phase of accelerated growth, driven by rising regulatory scrutiny, expanding pharmaceutical pipelines, and the rapid integration of artificial intelligence (AI) into drug safety monitoring systems.
Valued at approximately US$ 8.85 billion in 2026, the market is projected to reach around US$ 16.62 billion by 2032, growing at a robust compound annual growth rate (CAGR) of 10.3% during the forecast period from 2026 to 2032.
Pharmacovigilance plays a critical role in ensuring patient safety by detecting, assessing, monitoring, and preventing adverse drug reactions (ADRs) across the drug lifecycle. With the global rise in drug approvals, biologics, biosimilars, and specialty therapies, the volume and complexity of safety data have increased significantly. This has placed greater emphasis on comprehensive post-marketing surveillance and real-time safety monitoring, making pharmacovigilance an indispensable function for pharmaceutical, biotech, and healthcare organizations.
Pharmacovigilance plays a critical role in monitoring drug safety and managing adverse drug reactions across the pharmaceutical lifecycle.
Request Sample PDF Report: https://www.quintilereports.com/request-sample/583-pharmacovigilance-market/
One of the primary drivers of market growth is tightening global regulatory requirements. Regulatory authorities such as the US FDA and the European Medicines Agency (EMA) continue to strengthen mandates for adverse event reporting, benefit–risk assessment, and compliance across clinical and post-approval stages. These frameworks are pushing companies to invest in advanced pharmacovigilance systems capable of handling large datasets while ensuring accuracy, transparency, and regulatory alignment.
Technological advancements are transforming the pharmacovigilance landscape. The integration of AI, machine learning, and natural language processing (NLP) has significantly improved signal detection, automated case processing, and literature monitoring. AI-driven analytics enable faster identification of safety signals, predictive risk assessment, and improved decision-making, reducing manual workloads and operational costs. Additionally, cloud-based pharmacovigilance platforms are gaining traction, offering scalability, cross-border data sharing, and seamless global safety operations.
As regulatory expectations continue to evolve, pharmacovigilance is becoming increasingly proactive. Integration of real-world evidence, digital reporting tools, and global safety databases will further strengthen early risk detection and improve long-term patient outcomes worldwide.
From a regional perspective, North America holds the largest share of the pharmacovigilance market, accounting for approximately 40% of global revenue. This dominance is attributed to stringent regulatory frameworks, high levels of pharmaceutical activity, and early adoption of advanced digital safety technologies. Europe follows with around 30% market share, supported by mature regulatory systems, robust reporting mechanisms, and widespread outsourcing of pharmacovigilance services. Meanwhile, the Asia-Pacific region is expected to witness the fastest growth, driven by expanding pharmaceutical manufacturing, rising clinical trial activity, and increasing regulatory alignment in emerging economies such as China, India, and Southeast Asia.
Market dynamics are further shaped by strategic mergers and acquisitions. Leading pharmacovigilance and life sciences technology providers are actively acquiring AI-based analytics firms, cloud safety platforms, and NLP solution developers to enhance their technological capabilities and expand service portfolios. These strategic moves are aimed at delivering end-to-end pharmacovigilance solutions that cover case intake, signal detection, risk management, and regulatory reporting.
In terms of segmentation, contract outsourcing dominates the market, as pharmaceutical companies increasingly rely on specialized service providers and CROs to manage large volumes of safety data efficiently. Spontaneous reporting remains a core method for ADR detection, while real-world data sources such as electronic health records and social media monitoring are emerging as valuable tools for proactive risk management. Phase IV clinical trials represent the largest clinical segment, reflecting the growing focus on post-marketing surveillance and long-term drug safety.
Key players operating in the pharmacovigilance market include Cognizant, IBM, IQVIA, ArisGlobal, and BioClinica, among others. These companies leverage advanced analytics, global delivery models, and extensive healthcare data assets to maintain competitive advantage and meet evolving regulatory and industry demands.
Looking ahead, the pharmacovigilance market is set to benefit from continued digital transformation, growing emphasis on patient safety, and increasing use of real-world evidence. As AI-driven safety monitoring and regulatory harmonization gain momentum, pharmacovigilance will remain a cornerstone of modern healthcare systems, ensuring safer medicines and improved patient outcomes worldwide.
Our Latest Publication
Pharmacovigilance Market Size Estimation, Share & Future Growth Trends Analysis, By Product (Chemotherapy, Contract Outsourcing), By Application, By End Use, and Regional Analysis, 2024-2032
Our Latest News:
Automotive Composites Market Accelerates as Lightweighting, Electrification, and Sustainability Redefine Vehicle Design
Adarsh
Business Strategy — Quintile Reports
Adarsh is a Business Strategy professional focused on transforming market insights into actionable growth plans. He supports strategic initiatives through market analysis, competitive intelligence, and data-driven decision-making to help drive long-term business success.
His core skills include strategic planning, market research, growth opportunity assessment, trend analysis, performance tracking, stakeholder communication, cross-functional collaboration, and critical problem-solving.