The global Minimal Residual Disease (MRD) Testing Market is gaining momentum as oncology care increasingly shifts toward precision-driven diagnostics and personalized treatment strategies.
Advances in molecular testing and growing emphasis on early relapse detection are reshaping how clinicians monitor cancer treatment outcomes worldwide.
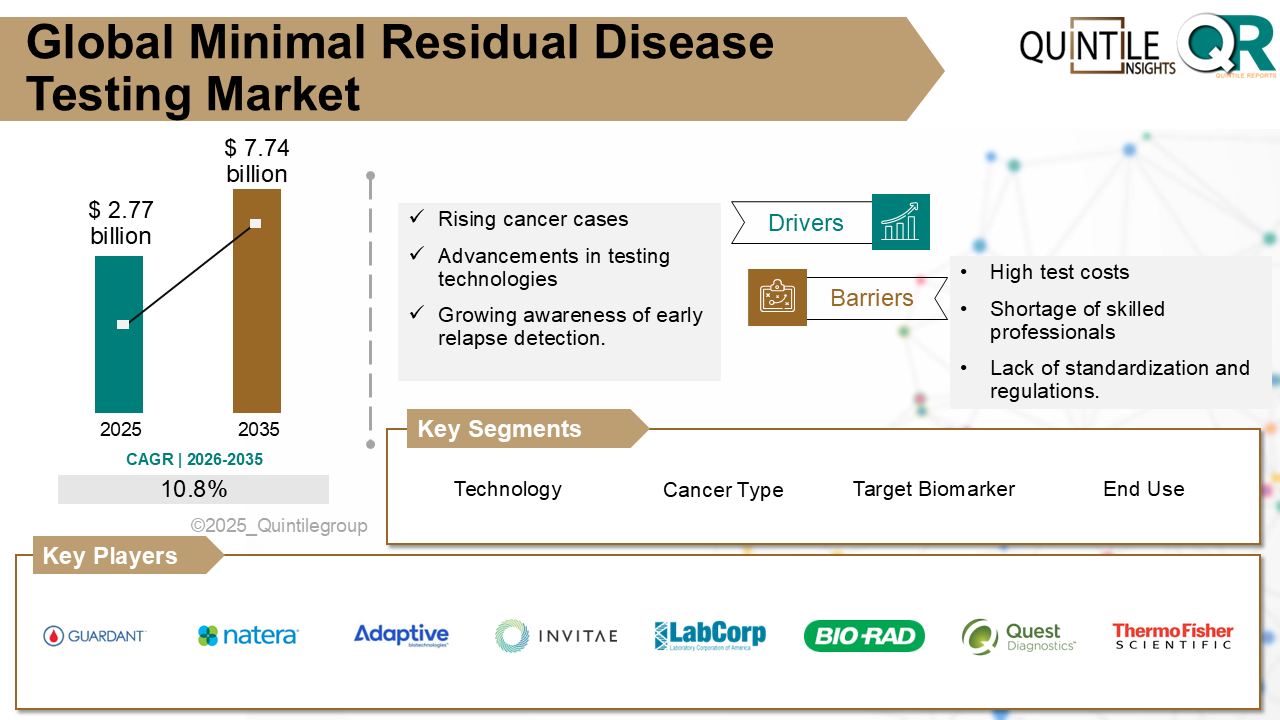
Valued at USD 2.77 billion in 2025, the market is projected to reach USD 7.74 billion by 2035, expanding at a compound annual growth rate (CAGR) of 10.8% during the forecast period from 2026 to 2035. This growth is driven by rising cancer incidence, rapid technological innovation, and increasing clinical recognition of minimal residual disease as a meaningful biomarker in cancer management.
Request Sample PDF Report: https://www.quintilereports.com/request-sample/1216-minimal-residual-disease-testing-market/
Minimal residual disease refers to the small number of malignant cells that remain in a patient’s body following treatment, even when remission is achieved through conventional diagnostic methods. These residual cells are often undetectable using standard imaging or laboratory tests but pose a significant risk of disease recurrence. Advanced MRD detection techniques—including next-generation sequencing (NGS), flow cytometry, and polymerase chain reaction (PCR)—enable highly sensitive identification and quantification of residual cancer cells. As a result, MRD testing has become a critical tool for evaluating treatment response and guiding therapy decisions, particularly in hematological malignancies.
Market Drivers and Technological Progress
One of the primary drivers supporting expansion of the Minimal Residual Disease (MRD) Testing Market is the rising global burden of cancer, especially blood cancers such as leukemia, lymphoma, and multiple myeloma, which carry a high risk of relapse. Clinicians are increasingly relying on molecular response monitoring to optimize treatment strategies and improve long-term outcomes. The broader adoption of precision and personalized medicine further strengthens demand for advanced MRD diagnostic solutions.
Technological innovation continues to play a central role in shaping market dynamics. Improvements in NGS platforms, digital PCR systems, and high-parameter flow cytometry have significantly enhanced detection sensitivity, accuracy, and turnaround times. These advancements have enabled MRD testing to transition from research-focused applications into routine clinical practice. In addition, MRD is gaining acceptance as a surrogate endpoint in oncology clinical trials, encouraging its integration into drug development programs.
Challenges Limiting Wider Adoption
Despite its strong clinical potential, the MRD testing landscape faces several challenges. High assay costs and inconsistent reimbursement policies remain key barriers, particularly for advanced sequencing-based tests. In many regions, MRD assays are still considered investigational, limiting payer coverage and slowing adoption beyond clinical trial settings.
Technical complexity also presents obstacles. Residual disease testing requires sophisticated laboratory infrastructure, validated bioinformatics pipelines, and specialized expertise to interpret low-frequency variants. This limits accessibility in low-resource healthcare settings. Furthermore, the absence of standardized testing methodologies and reporting criteria complicates cross-study comparison and clinical decision-making.
Regional and Competitive Landscape
From a regional perspective, North America leads the Minimal Residual Disease (MRD) Testing Market, supported by advanced healthcare infrastructure, favorable reimbursement frameworks, and strong presence of diagnostic innovators. Europe follows closely, driven by robust public healthcare systems, research funding, and integration of molecular monitoring into oncology guidelines.
The Asia-Pacific region is expected to witness the fastest growth, fueled by rising cancer prevalence, expanding healthcare investments, and government-backed precision medicine initiatives in countries such as China, Japan, and India. Emerging markets in Latin America and the Middle East & Africa are also showing gradual adoption as diagnostic access improves.
Competition remains intense, with key players such as Natera, Guardant Health, Adaptive Biotechnologies, Roche, Bio-Rad Laboratories, Thermo Fisher Scientific, Illumina, and QIAGEN focusing on assay sensitivity, liquid biopsy platforms, and expanded clinical applications.
Outlook
Looking ahead, the Minimal Residual Disease (MRD) Testing Market is positioned for sustained long-term growth as cancer care continues to emphasize early relapse detection, minimally invasive diagnostics, and personalized treatment planning. Continued investment in technology development, reimbursement support, and standardization efforts will be essential to unlocking broader clinical and commercial adoption worldwide.
Our Latest Publication
Minimal Residual Disease Testing Market Size Estimation, Share & Future Growth Trends Analysis, By Technology (Flow Cytometry, Polymerase Chain Reaction (PCR), Next Generation Sequencing (NGS), Others), By Cancer Type, By Target Biomarker, By End Use and Regional Analysis, 2026-2035
Our Latest News:
Meniscal Tear Repair Transform Knee Injury Treatment
Adarsh
Business Strategy — Quintile Reports
Adarsh is a Business Strategy professional focused on transforming market insights into actionable growth plans. He supports strategic initiatives through market analysis, competitive intelligence, and data-driven decision-making to help drive long-term business success.
His core skills include strategic planning, market research, growth opportunity assessment, trend analysis, performance tracking, stakeholder communication, cross-functional collaboration, and critical problem-solving.