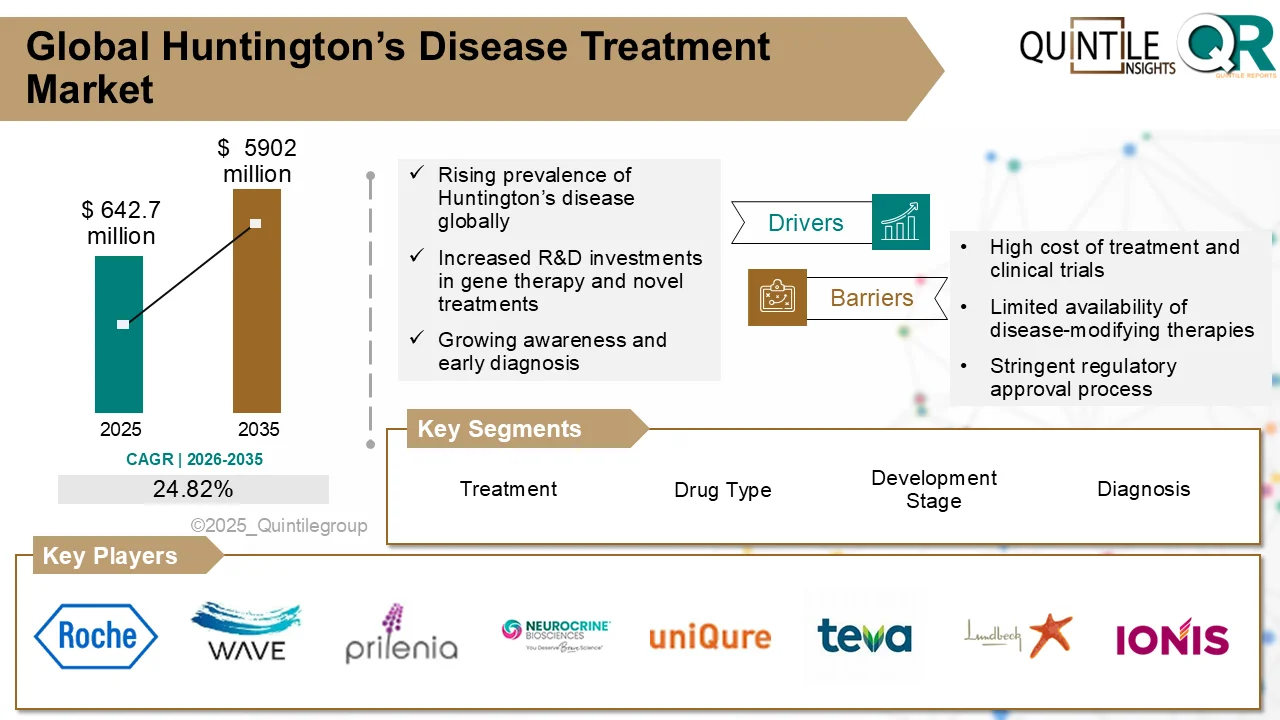
The global Huntington’s Disease (HD) Treatment Market is entering a transformative phase, driven by rapid advancements in therapeutic innovation, increasing diagnosis rates, and strong regulatory
support for rare disease drug development. According to Quintile Research, the market was valued at USD 642.7 million in 2026 and is projected to reach USD 5,902 million by 2035, registering a robust compound annual growth rate (CAGR) of 24.82% during the forecast period from 2026 to 2035.
Huntington’s disease is a rare, inherited neurodegenerative disorder characterized by progressive motor dysfunction, cognitive decline, and psychiatric symptoms. While no definitive cure exists, treatment strategies have evolved significantly, focusing on symptom management, disease progression control, and emerging gene-based interventions. The growing understanding of the disease’s genetic and molecular mechanisms is reshaping the competitive and clinical landscape.
Request Sample PDF Report: https://www.quintilereports.com/request-sample/1116-huntingtons-disease-treatment-market/
Market Growth Drivers
One of the primary drivers of market expansion is the rising prevalence of diagnosed Huntington’s disease cases, supported by improved access to genetic testing and increased awareness initiatives. Enhanced diagnostic protocols, particularly in North America and Europe, have significantly expanded the addressable patient population. Since 2020, diagnosis rates have increased substantially, creating sustained demand for both symptomatic and investigational therapies.
Another major growth catalyst is the rapidly advancing research pipeline. More than 20 clinical trials are currently underway, targeting various aspects of disease pathology. These include gene-silencing therapies such as antisense oligonucleotides (ASOs), RNA interference (RNAi), and emerging CRISPR-based gene-editing approaches, along with neuroprotective agents designed to address underlying neuronal damage. A large proportion of these candidates benefit from orphan drug designation, accelerating clinical development and regulatory review.
Special Discount: https://www.quintilereports.com/request-discount/1116-huntingtons-disease-treatment-market/
Strategic collaborations between biotechnology firms, academic institutions, and patient advocacy organizations are also strengthening innovation ecosystems. Increased government funding, including grants from organizations such as the NIH, along with rising private investments, has reduced development timelines and improved trial efficiency.
Regulatory and Competitive Landscape
Regulatory authorities play a critical role in shaping the HD treatment market. Agencies such as the U.S. FDA and the European Medicines Agency are offering fast-track approvals, breakthrough therapy designations, and extended market exclusivity for rare disease treatments. These incentives have significantly increased the number of therapy applications and encouraged pharmaceutical companies to invest in high-risk, high-reward research.
Place Order: https://www.quintilereports.com/request-enquiry/1116-huntingtons-disease-treatment-market/
The competitive environment is evolving rapidly, with established pharmaceutical companies and emerging biotech firms competing across both symptomatic and disease-modifying segments. While VMAT2 inhibitors remain the standard of care for managing chorea, innovation is increasingly centered on therapies that target the mutant huntingtin protein at the genetic level.
Recent developments highlight this shift. In late 2024, Novartis entered a high-value licensing agreement for an oral splice-modulator designed to reduce mutant huntingtin protein levels, demonstrating promising clinical outcomes. In 2025, uniQure’s gene therapy candidate AMT-130 received Breakthrough Therapy designation following positive trial results showing meaningful slowing of disease progression.
Regional Trends
North America dominates the global market, supported by advanced healthcare infrastructure, favorable reimbursement frameworks, and a strong clinical research ecosystem. The U.S. leads in gene-based therapy development and patient advocacy, contributing to early diagnosis and treatment adoption.
Europe represents the second-largest market, characterized by centralized care models, national patient registries, and strong government support for rare diseases. Countries such as Germany and France play a key role in clinical research and therapy access.
The Asia-Pacific region is emerging as the fastest-growing market, driven by healthcare modernization, improved diagnostic access, and increasing government initiatives focused on rare disease management. China and Japan are witnessing growing participation in clinical research and therapeutic adoption.
Outlook
Despite challenges such as high treatment costs, limited patient populations, and complex clinical trial requirements, the Huntington’s disease treatment market is positioned for sustained growth through 2035. Ongoing investments in research and development, combined with regulatory incentives and scientific breakthroughs, are expected to redefine treatment paradigms.
As innovation shifts from symptom management toward disease modification, the Huntington’s Disease Treatment Market is set to become one of the most dynamic segments within the rare neurological disorders space.
Q1. What is the Huntington’s Disease Treatment Market?
Answer:
The Huntington’s Disease Treatment Market comprises therapies, drugs, and supportive care solutions used to manage Huntington’s disease, a rare inherited neurodegenerative disorder. The market includes symptomatic treatments, disease-modifying therapies, gene-based treatments, and palliative care aimed at improving patient quality of life.
Q2. What is the current size of the Huntington’s Disease Treatment Market?
Answer:
According to Quintile Research, the global Huntington’s Disease Treatment Market was valued at USD 642.7 million in 2026 and is projected to reach USD 5,902 million by 2035, growing at a CAGR of 24.82% during the forecast period from 2026 to 2035.
Q3. What factors are driving the growth of the Huntington’s Disease Treatment Market?
Answer:
Key growth drivers include increasing diagnosis rates due to improved genetic testing, rising awareness of rare neurological disorders, strong investments in research and development, expanding clinical trial pipelines, and regulatory incentives such as orphan drug designations and fast-track approvals.
Q4. What types of treatments are available for Huntington’s disease?
Answer:
Currently available treatments primarily focus on symptom management and include VMAT2 inhibitors, antipsychotics, antidepressants, mood stabilizers, and anticonvulsants. Emerging treatments under development include gene-silencing therapies, RNA-based therapies, and gene therapy approaches targeting the underlying genetic cause of the disease.
Q5. Are there any disease-modifying therapies for Huntington’s disease?
Answer:
As of now, there are no fully approved disease-modifying therapies that can halt or reverse Huntington’s disease progression. However, several promising candidates—including antisense oligonucleotides (ASOs), RNA interference therapies, and gene therapies—are in advanced clinical development stages
Q6. Which region dominates the Huntington’s Disease Treatment Market?
Answer:
North America dominates the global market due to advanced healthcare infrastructure, high diagnosis rates, strong patient advocacy, favorable reimbursement policies, and active participation in clinical research and gene-based therapy development.
Q7. Why is the Asia-Pacific region experiencing rapid market growth?
Answer:
The Asia-Pacific region is witnessing rapid growth due to improving healthcare infrastructure, increased access to genetic testing, rising awareness of rare diseases, government initiatives supporting orphan drugs, and expanding participation in clinical trials, particularly in China and Japan.
Q8. What are the major challenges restraining market growth?
Answer:
Key challenges include the lack of approved disease-modifying therapies, high treatment and development costs, small patient populations, adverse effects associated with existing drugs, complex clinical trial designs, and lengthy regulatory approval processes.
Q9. Who are the key players in the Huntington’s Disease Treatment Market?
Answer:
Major players include Roche, Pfizer, Teva Pharmaceutical Industries, Wave Life Sciences, Prilenia Therapeutics, Neurocrine Biosciences, uniQure, H. Lundbeck A/S, Ionis Pharmaceuticals, Sage Therapeutics, Novartis, Vertex Pharmaceuticals, and other emerging biotech companies.
Q10. What role do orphan drug designations play in this market?
Answer:
Orphan drug designations provide incentives such as tax credits, reduced regulatory fees, expedited approvals, and market exclusivity. These benefits significantly encourage pharmaceutical companies to invest in Huntington’s disease therapies despite the small patient population.
Our Latest Publication
Huntingtons Disease Treatment Market (2026 – 2035)
Our Latest News:
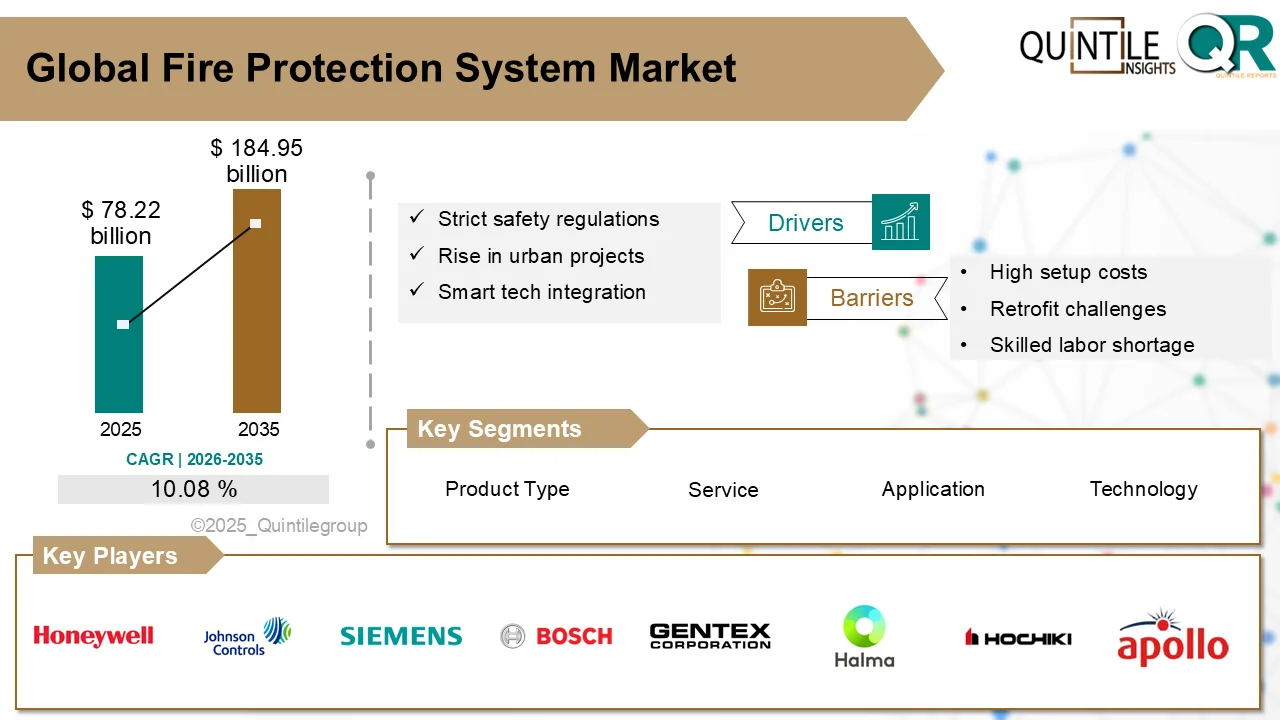
Fire Protection System Market Set for Robust Growth Through 2035, Driven by Smart Safety Technologies
Adarsh
Business Strategy — Quintile Reports
Adarsh is a Business Strategy professional focused on transforming market insights into actionable growth plans. He supports strategic initiatives through market analysis, competitive intelligence, and data-driven decision-making to help drive long-term business success.
His core skills include strategic planning, market research, growth opportunity assessment, trend analysis, performance tracking, stakeholder communication, cross-functional collaboration, and critical problem-solving.